Research
Overview
The lower urinary tract (LUT) includes the bladder and urethra in females plus the prostate and seminal vesicles in males. Diseases of the LUT including infection, dysfunction and cancer are poorly understood at the molecular level. The main goals of the Strand lab are to create comprehensive cellular atlases of healthy human and mouse LUT, characterize the molecular and cellular alterations in human disease, and build appropriate models.
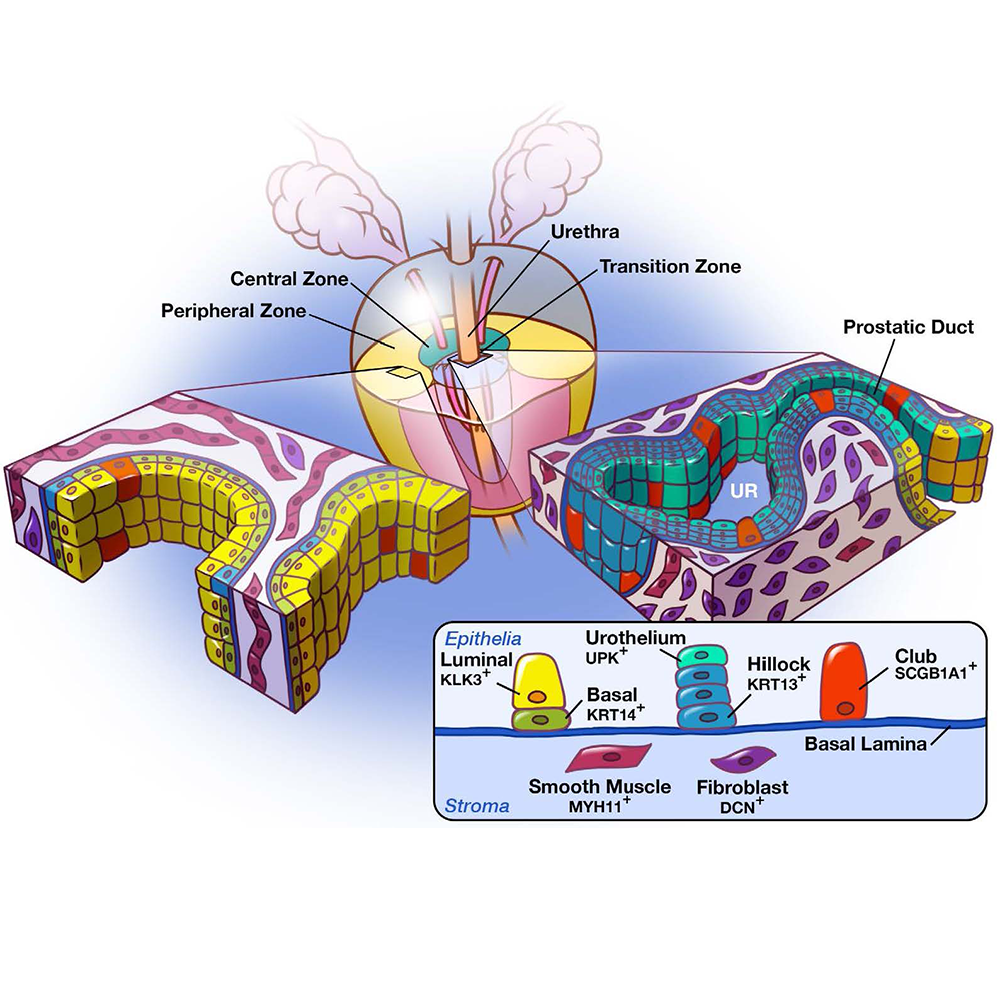
A Cellular Anatomy of Human and Mouse LUT
Our cellular atlases are made publicly available as CZ CELLxGENE Datasets. These can be explored interatively in a web browser, or downloaded for further exploration. Below you can find links to our collections on CELLxGENE.
- Single cell analysis of mouse and human prostate reveals novel fibroblasts with specialized distribution and microenvironment interactions
- Urethral luminal epithelia are castration-insensitive cells of the proximal prostate
- A Cellular Anatomy of the Normal Adult Human Prostate and Prostatic Urethra
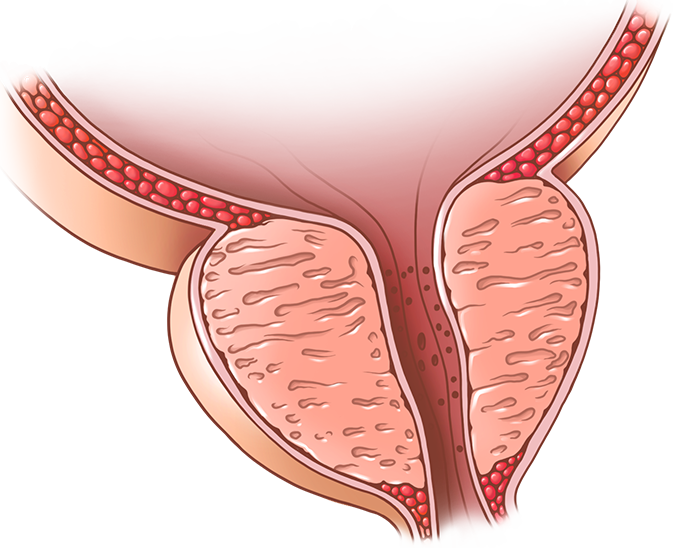
Benign Prostatic Hyperplasia (BPH)
BPH and associated lower urinary tract symptoms (LUTS) are present in a majority of men over 70 years old resulting in the need for medical treatment or surgical intervention. Clinical trials of medications for BPH demonstrate that personalized therapies will be needed due to highly variable responses to individual drugs. Typically, patients complaining of lower urinary tract symptoms (LUTS) including nocturia, urgency and frequency are placed on an alpha-adrenergic receptor blocker as a first line therapy. Alpha blockers relieve smooth muscle tension and are most effective in patients with early stage disease displaying a high smooth muscle to epithelial prostate tissue composition. However, as the prostate enlarges, epithelial nodules predominate the tissue decreasing the likelihood of alpha-blocker efficacy. As the prostate enlarges (typically 2-3% per year), 5 alpha reductase inhibitors (5ARI) are administered as a second line therapy and work by blocking production of dihydrotestosterone (DHT). 5ARIs reduce prostate volume ~25% after 6-9 months treatment and slow the worsening of LUTS. Neither alpha blockers nor 5ARIs are a cure, because these therapies target the symptoms rather than the molecular drivers of BPH. Our goal is to provide a better molecular and cellular characterization of BPH to develop new therapies focused on molecular drivers of prostate growth.